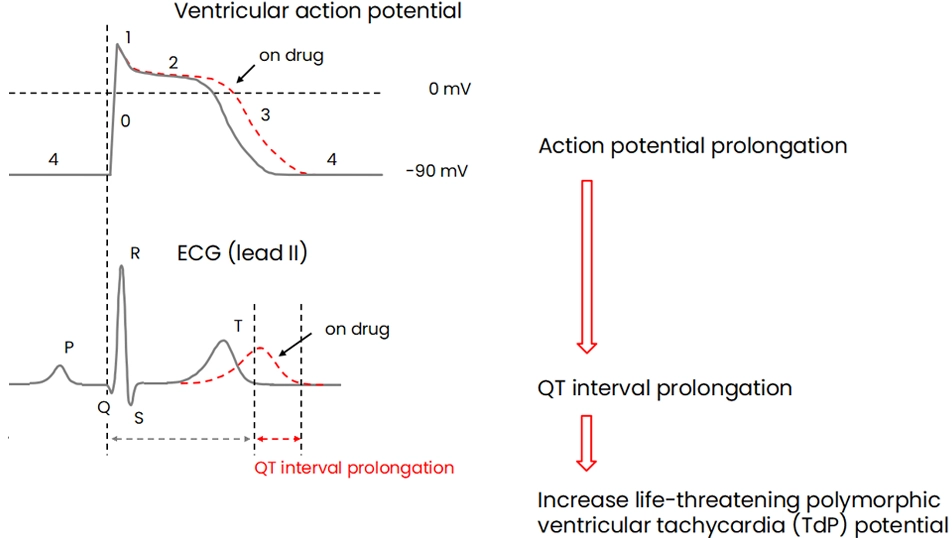
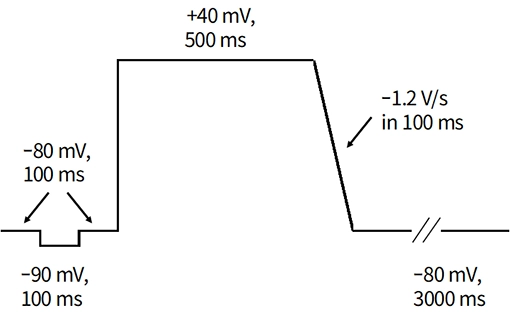
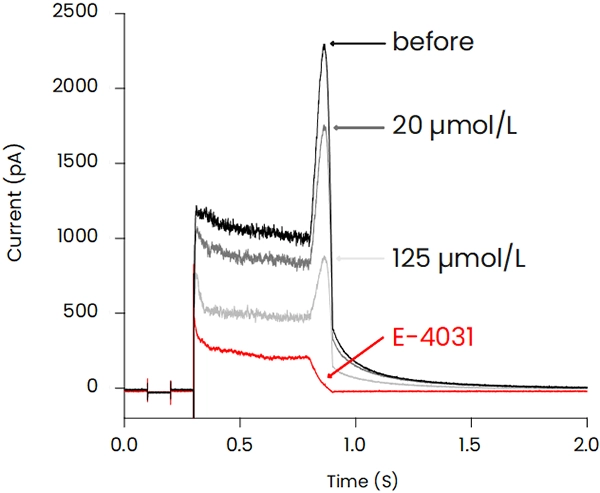
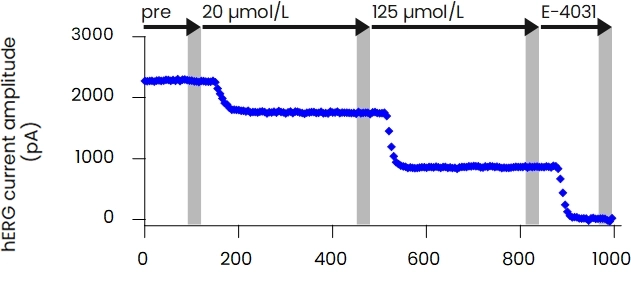
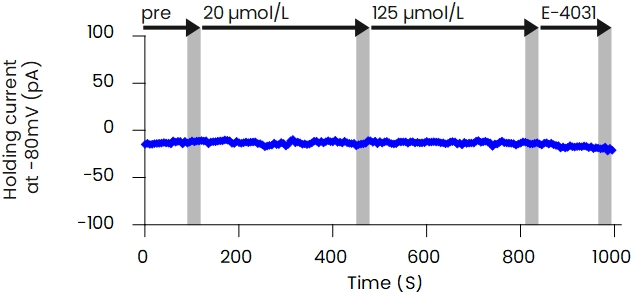
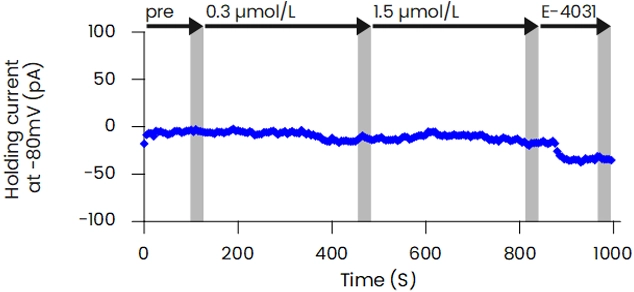
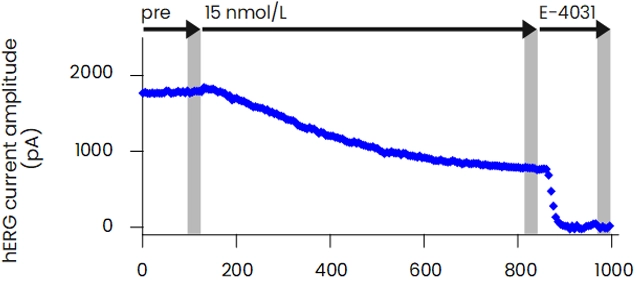
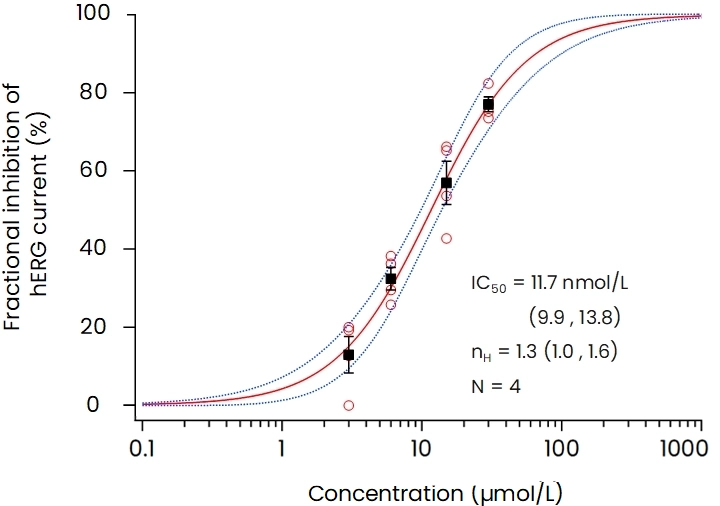
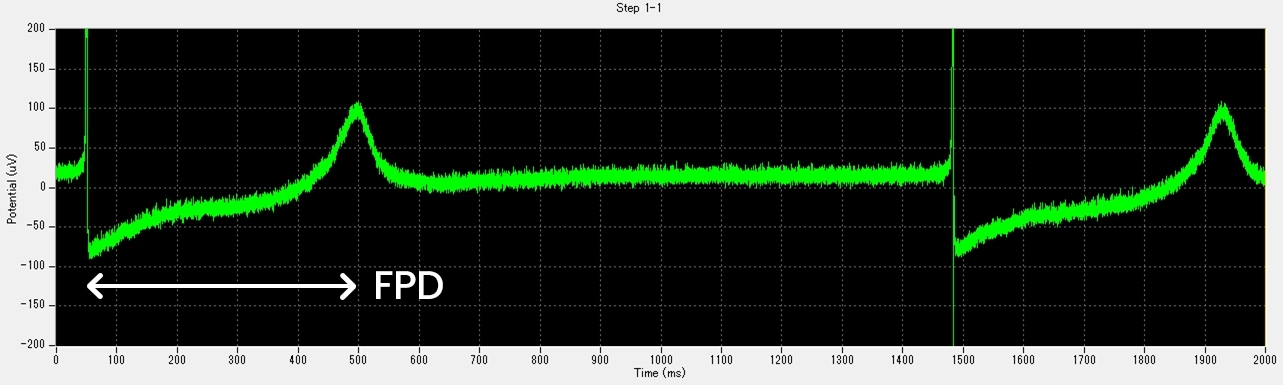
The patch-clamp method, a technique for measuring cellular electrical activity, has been widely used in a variety of fields such as basic research and drug discovery. Mediford mainly provides study services for evaluating the effects of drugs on cardiac ion channels using cardiac ion channel-expressing cells and the accurately measurable manual patch-clamp platform. We offer evaluations from a wide range of implementation standards, from screening to GLP. Furthermore, we can also conduct studies under customized conditions and develop assay systems according to our clients’ needs.
Mediford always strives to improve the quality of our patch-clamp assay services and to ensure our services are tailored to meet our clients’ needs. Please feel free to contact us.
Click here to contact us